| Balancing Heat && Cold |
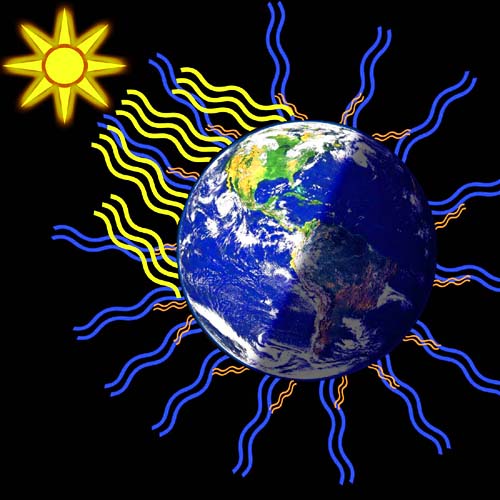
| Chapter 3: “The Chemistry of Global Warming” The average temperature of the Earth is 15°C (59°F). This is cool but not cold. It's the temperature of a nice spring or fall day. It keeps this average temperature because there's a balance of heat gain from the Sun(yellow lines), heat being lost to the freezing cold of outer space (blue lines), and heat being returned to the Earth by the atmosphere (orange lines). If the Sun's heat is blocked, the Earth can freeze quickly. If something keeps in the heat too much, Earth can also heat up quickly, too. | |
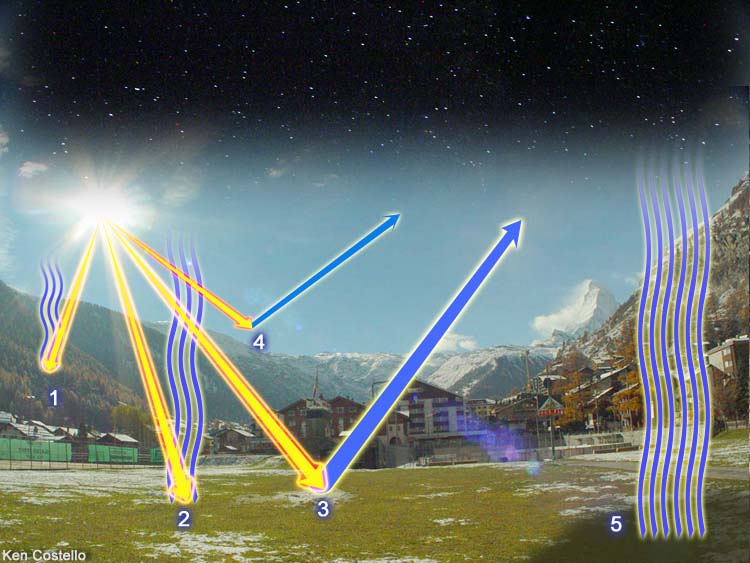
Follow the paths that energy takes... | |
| Energy from the sun follows different paths. Starting with #1 ray of light, we see it entering the atmosphere and being absorbed by the air. Some of this heat radiates back to space. The #2 ray of sunlight hits the ground and heats the ground. Some of this heat radiates back up to space. The #3 ray reflects off the ground and goes back into space. The #4 ray gets reflected back to space by the atmosphere. Even where the sun isn't shining, heat is radiating out to space. In other words, a lot of energy hits the Earth but the Earth either reflects the heat or radiates heat back into space. Without some way of returning this heat going up into space, the Earth would be a frozen wasteland. Fortunately, we have some gases in the air which intercept this energy and radiate much of it back to the ground. | |
 | |
| Naturally occurring gases like ozone, water vapor, methane, and carbon dioxide let the visible light pass as it comes from the sun and goes through the atmosphere. However, when the heat energy (infrared light) heads back into space, these gases absorb (trap) that energy. They then radiate about 84% of it back to the Earth's surface. The lines around carbon dioxide represent the vibration that all of these molecules do that causes them to absorb the infrared radiation (heat) and re-radiate in all directions. The orange arrows show the direction that sends it back to the Earth's surface. This makes Earth warmer and inhabitable. However, humans are adding other gases into the air like freon and nitrogen oxide; plus, we are adding additional amounts of ozone, methane, carbon dioxide, and water. These intercept more heat sending more back to Earth's surface. This is how global warming is explained. We have too many molecules capturing the heat that use to go out into space. It's like the Earth is wearing an extra coat that it didn't have on before and doesn't want now. | |
 | Carbon Dioxide: Carbon dioxide is the building block for all plant growth. However, too much in the atmosphere is believed to be the leading cause of global warming. Looking at samples of ice dating back 160,000 years gives us some clues that CO2and world temperature are connected. Samples of ice are taken with a core drill (see below) |
 Left photo: Mark Twickler, University of New Hampshire. Top photo: Lonnie Thompson, Ohio State University. Bottom right photo: Kendrick Taylor, Desert Research Institute. | |
A drill is set up on the ice which cuts out a cylinder from the ice. As the drill moves down, the ice core slides into a metal pipe. The pipe is pulled out of the drill hole and the ice is carefully removed from the metal pipe. The ice core is stored in wood trays at freezing temperatures. Examining the ice cores gives you a history of what happened in the atmosphere for hundreds and thousands of years. Bubbles in the ice trap prehistoric atmosphere, which can be analyzed. Below is a graph of the CO2 from the bubbles over the last 160,000 years taken from the textbook on page 97. It shows a correlation between temperature and the amount of CO2 in the air. | |
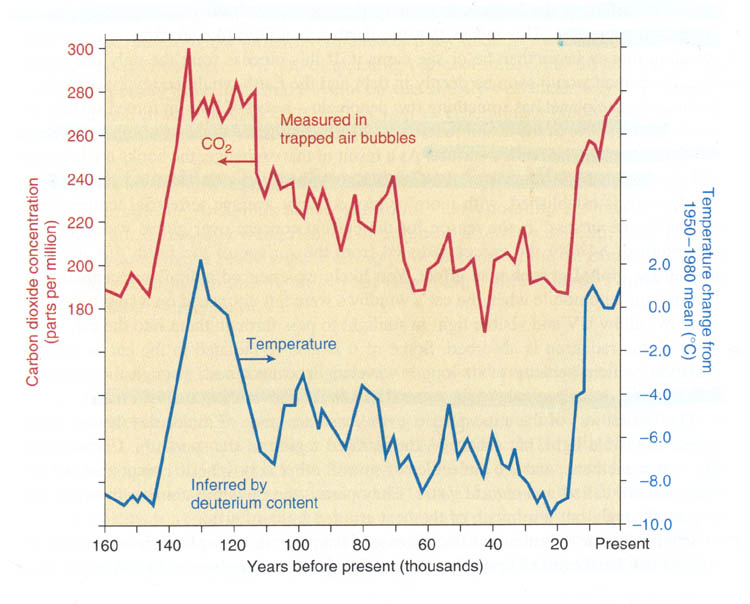 | |
This graph shows that when CO2 levels were high the calculated temperature was also high. It appears that 130,000 years ago there was a high level of CO2 and the temperature was higher as well (Why is was high I'm not sure). In the last 15,000 years it appears we are on an upswing again. So global warming might be a natural cyclic process. Unfortunately, since we are on the upswing, adding more CO2 in the atmosphere may be the wrong time to add more heat. If we were to produce more CO2 30,000 years ago, it may have helped us get out of the ice age. Notice the temperature difference from 20,000 years ago to present is a different of about 10°C or 18F. That's the difference between a day where water is always frozen (32°F) to a cool 50°F long sleeve shirt day. Or you can look at it as a hot Phoenix summer day of 118°F turning into 140°F where all plants die, all cars overheat, and air conditioners can't cope. So 18°F (10°C) makes a big difference in the world. More alarming is that in the above graph we can find jumps of 50 parts per million of CO2 but it took at least 10,000 years. We've had a jump of almost 50 parts per million in the last 40 years. See the graph below. | |
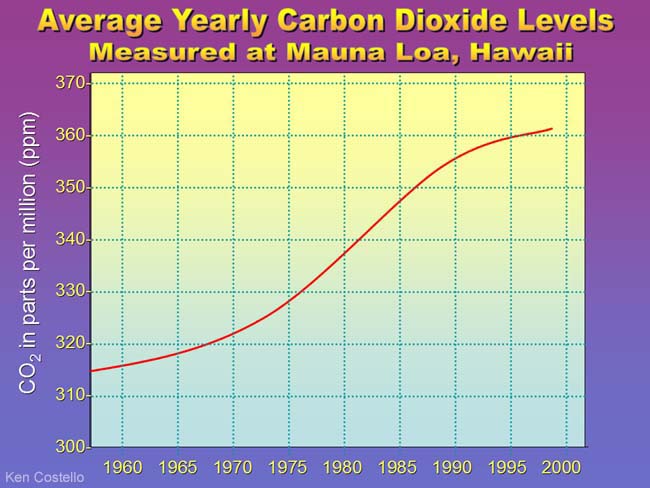 | |
The air measured at the top of Mauna Loa volcano in Hawaii is thought to be representative of the average CO2 levels throughout the world. In 1957 CO2 levels were around 315 CO2 molecules out of every 1,000,000 air molecules & atoms (315 ppm). By the year 2000 the CO2 levels were up to above 360 ppm. Before the industrial revolution and before man started using coal to fuel all the factories, carbon dioxide levels were about 280 ppm. The last 40 years shows a rise of about 50 parts per million in CO2 levels; as much as nature took 10,000 years to do. We've been fortunate that this increase has caused some increase in temperature (about 0.5°F) but not the jump in temperatures that Earth experienced before when CO2 jumped that much (about 7°C or 13°F). So where does the CO2 come from? (see below) | |
Carbon Cycle 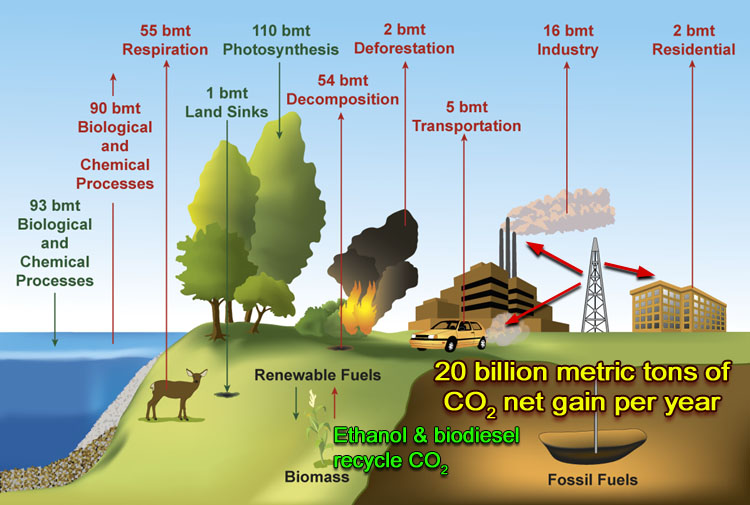 | |
| This graphic illustrates what happens to carbon including carbon dioxide. The "bmt" units stand for billion metric tons. A metric ton is 2,200 lbs., about the weight of a compact car. One billion metric tons would be the weight of 1,000,000,000 compact cars. | |
Absorbs CO2 | Gives off CO2 |
Let's start from the left. 93 bmt would the weight of 93,000,000,000 compact cars. This is what the ocean absorbs. Ocean plants use CO2 to grow and many sea creatures use it to grow shells. | The ocean also gives off 90 billion metric tons, in part from all of the animals that live in the ocean. |
| Plants absorb 110 billion metric tons as they grow. In photosynthesis CO2 is turned to sugar molecules. Here's the simplified reaction: 6H2O + 6CO2 + light -> C6H12O6 (glucose) + 6O2 Glucose is utilized as a building block for the rest of the plant. | Animals on land (including us) place about 55,000,000,000 metric tons of CO2 in the air. Each person's own breath contributes about 1,000 lbs or CO2 to the atmosphere each year. We basically do the reverse reaction of photosynthesis:C6H12O6 (glucose) + 6O2 -> 6H2O + 6CO2+ Energy |
 | Decomposition of dead animals and plant material gives off 54 billion metric tons of carbon as methane and as carbon dioxide. |
 | The burning of fossil fuel (petroleum) for energy in factories and automobiles and for heating homes and businesses contribute 23 billion metric tons. If you drive 12,000 miles per year and your car gets 20 miles per gallon, your car contributes 12,000 lbs (5 metric tons) of CO2 per year (1 gal gasoline yeilds 20 lbs CO2). |
 Photo: USDA Forest Service | Clearing of forests and burning of forests (mostly in Brazil) contributes 2 billion metric tons. |
| Total CO2 Absorbed Yearly = 204 billion metric tons | Total CO2 put into atmosphere = 224 billion metric tons (weight 224 billion cars) |
| It's this excess of 20 billion metric tons (44 trillion lbs) of CO2 that is added to the atmosphere every year that is most blamed for the current global warming. | |
 | |
| "Hi, Earth. I like your wool cap, but don't you think it's a little too hot to be wearing that? From the frown you're making, I'd say you were a little stressed." | |
No comments:
Post a Comment